Through its Vaccine Sealing Center, LogVett supports the government in the control process of rabies vaccines for herbivores. At the end of September, the Ministry of Agriculture, Livestock, and Supply (MAPA) initiates the Harvesting process at LogVett, in which the regulatory body samples vaccines manufactured by industries for official testing to release them to the market.
This verification is done through a comparative analysis with the National Reference Vaccines (VRN), the so-called double check, since industries also internally test the products for market release, being essential to attest the quality of products sold in the national market.
The new batch of VRNs arrived at LogVett this second semester. The importation was requested by MAPA and carried out by veterinary product industries, represented by the National Syndicate of the Industry of Products for Animal Health (Sindan), in a public-private partnership.
The VRNs are stored in LogVett’s Vaccine Sealing Center, as well as those manufactured by the industry to meet the market demand. During the harvesting process, samples are taken for submission to the regulatory body, while a similar amount is retained as a control sample, in case retesting is needed. The rest of the manufactured vaccines remain in quarantine in the warehouse, awaiting approval to be commercialized.
Once approved, they leave quarantine and the sealing process begins at LogVett, in which each vaccine vial receives a holographic seal for product traceability. Only after this process are the vaccines distributed to the market.
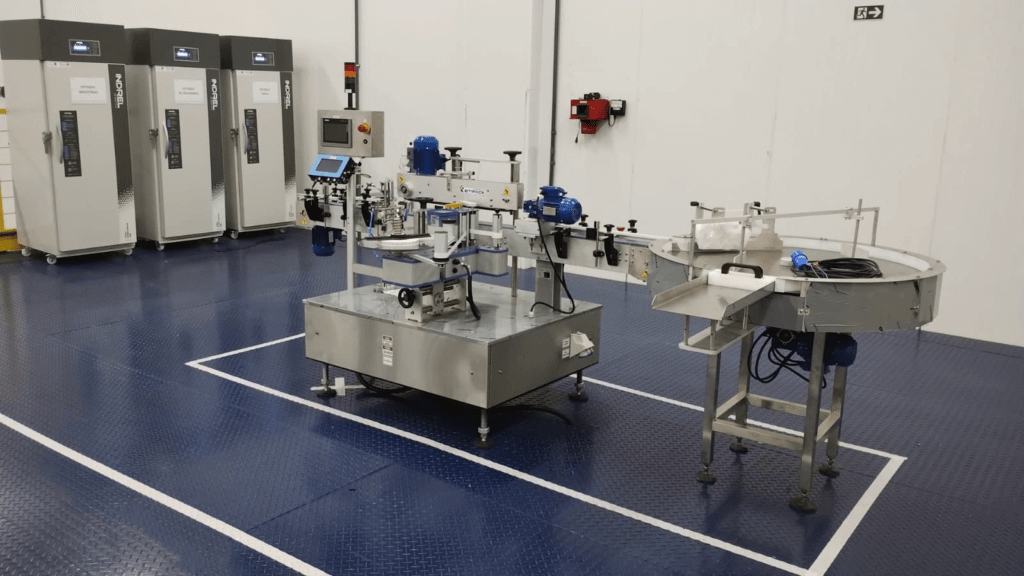
“LogVett is structured and accredited to perform sealing for other vaccines requiring control and traceability,” explains Fernando Palata, a veterinary technician responsible for overseeing the entire harvesting and sealing process, which, along with industry technicians, ensures the traceability of the process.
“It is a source of pride for LogVett to support the government in such an important process,” comments Mauricio Motta, CEO of LogVett. “We have set up a Sealing Center with state-of-the-art equipment aiming for more productivity and safety in the process.”
LogVett also supports industries in the distribution process, ensuring that products are transported under the appropriate temperature conditions to guarantee the stability of the vaccines.
Transport is carried out in refrigerated vehicles or in styrofoam boxes designed to maintain a temperature of 2°C to 8°C for up to 48 hours. When the distance traveled exceeds this period, the ice is renewed before the deadline at support points to ensure it arrives in perfect condition to the consumer.
“We are committed and focused on serving the Animal Health market by offering a high level of service and specialization in logistics for this segment in Brazil,” concludes Motta.